Legal aspects of gathering and using biological material for genetic research
Contents
- 1 Legal Requirements for Provenience, Transfer, Access and Utilization of Genetic Resources under consideration of the Nagoya-Protocol
- 2 General information for scientists
- 3 Information on the National Focal Point and other relevant parties in Germany
- 4 Information provided by the Deutsche Forschungsgemeinschaft (DFG)
- 5 Information provided by the Nationale Forschungsdateninfrastruktur (NFDI)
- 6 Attention!
Legal Requirements for Provenience, Transfer, Access and Utilization of Genetic Resources under consideration of the Nagoya-Protocol
The Nagoya Protocol (NP) is an international agreement that entered into force on 12 October 2014. It aims at sharing the benefits resulting from the utilization of genetic resources in a fair and equitable way. The implementation of the Nagoya Protocol went into force across the EU Member States in October 2014 (EU Regulation 511/2014). Germany has implemented the EU Regulation 511/2014, since 1st of July 2016 it is effective.
The requirements for provenience, transfer, access and the utilization of genetic resources (Nagoya Protocol) do also apply for basic scientific research. Data being archived and/or processed by GFBio need to be compliant to the implemented relevant national and international regulations.
Genetic material that was collected prior to December 29, 1993 is not subject of international laws on biodiversity.
General information for scientists
- The Nagoya Protocol HuB aims to help academic researchers in Germany with understanding Nagoya Protocol compliance.
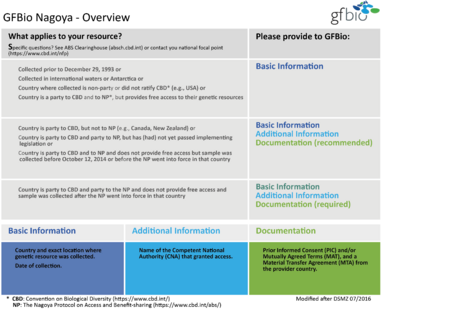
- The Nagoya-Chart at the right is based on a chart set up by the DSMZ. The diagram intends to guide DFG data producers to check the legal requirements before starting their research study on genetic material.
- The Convention on Biological Diversity (CBD) provides a list of parties incl. individual status of ratification of the Nagoya Protocol
- For international advice please consult the ABSCH - international platform exchanging information on ABS
- EU Regulation 511/2014
Information on the National Focal Point and other relevant parties in Germany
- Federal Agency for Nature Conservation (BfN) Info: Nagoya-Protokoll (Nutzung genetischer Ressourcen)
Nagoya Protocol on Access and Benefit-sharing and ABS National Focal Point Dr. Stefan Lütkes Division N II 1, Nature Conservation and Landscape Management Legislation Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU) Robert Schumann Platz 3 53175 Bonn, Germany +49 228 99 305 2670 +49 228 99 305 2694 stefan.luetkes@bmu.bund.de
ABS Competent National Authority Federal Agency for Nature Conservation Konstantinstr. 110 53179 Bonn, Germany +49 228.8491.1311 Nagoya-CNA@bfn.de
Information provided by the Deutsche Forschungsgemeinschaft (DFG)
- Ergänzender Leitfaden für die Antragstellung von Forschungsvorhaben, die unter das Übereinkommen über die biologische Vielfalt (Convention on Biological Diversity-CBD) fallen
- Erläuterungen zu Forschungs- und/oder Entwicklungsvorhaben, die Zugang zu genetischen Ressourcen und/oder zu traditionellem Wissen, das sich auf genetische Ressourcen bezieht, beinhalten (Veröffentlichung der ständigen Senatskommission für Grundsatzfragen der biologischen Vielfalt 12/2019)
- Model Clauses for Mutually Agreed Terms on Access to Genetic Resources and Benefit Sharing (Veröffentlichung der ständigen Senatskommission für Grundsatzfragen der biologischen Vielfalt 12/2019)
Information provided by the Nationale Forschungsdateninfrastruktur (NFDI)
- The lecture and talk "What has the Nagoya Protocol got to do with NFDI?" (NFDI InfraTalk 12.04.2021) gives an inside view of the implication that (upcoming) international legal regulations (might) have on data science, open data and exchange of scientific information.
Attention!
Scientists have to check the ABS (Access and Benefit Sharing) requirements before starting their research study. The GFBio data centers and all Natural Science Collections follow the CETAF Recommendations concerning ABS with the CETAF Code of Conduct and Best Practices. Thus they must ask researchers for permits and appropriate documentation before accepting biological material and data based on that material.